COVID Vaccines
The CDC recommends everyone 12 years of age and older should get a vaccination to help protect against COVID-19.
The vaccine is free of charge to everyone living in the United States, regardless of their immigration or health insurance status.
Some organizations may bill insurance for the administration fee but there should be no out-of-pocket expense regardless of coverage.
KDMC is administering the MODERNA vaccine to adults 18 years and older while offering PFIZER vaccines for children between 12-17 years of age.
See link below for side effects:
To help mediate side effects, take ibuprofen or acetaminophen immediately after your vaccine.
Vaccines are readily available, click on the link to find a location.
We still need to wear masks and practice social distancing.
Vaccines are one important step in our fight to control COVID-19, but we
still need to be mindful and dutiful in all we do if we want to end this pandemic.
This means it's still important to adhere to the following safety measures:
- Get Vaccinated.
- Wear a mask.
- Stay 6 feet apart from others whenever possible.
- Wash your hands.
- Stay home if you're sick
Schedule Your Vaccination
-
KDMC Vaccine Hotline
601.835.9239
Vaccine Hotline
-
MSDH COVID-19 Vaccine Hotline
877.978.6453
COVID-19 Booster Shot
At this time, the COVID-19 booster shot for Moderna and Pfizer has been approved by the CDC and the Mississippi State Department of Health. This is for patients who fit defined immunocompromised categories listed in the link below. If you are a KDMC Physician Clinic patient, you can inquire with your KDMC provider to determine if you are a candidate for the Moderna booster vaccine. KDMC is not currently administering the Pfizer booster shot.
If you are not a patient of KDMC Physician Clinics, we recommend you inquire from your primary care provider and contact one of the vaccine locations listed below.
Vaccines.gov - Search for COVID-19 vaccine locations
Who Should Get It?
As a community and state, the availability of a safe and effective vaccine is an exciting breakthrough in our ability to return to the lives we once knew and protect our families and loved ones.
The Centers for Disease Control and Prevention (CDC), Mississippi Department of Health (MSDH) and Human Services (MSDHS), and King’s Daughters Medical Center recommend all those who are 18 years of age and older receive the COVID-19 vaccine regardless of their prior infection status. We pledge equal access no matter race, ethnicity, or economic status, and vaccine administration details will be shared widely, once confirmed.
COVID Vaccine Information for Women
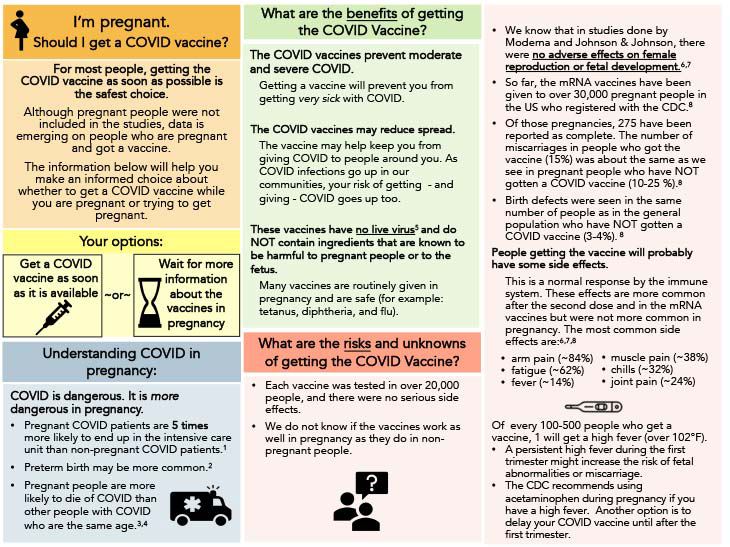
In an effort to clarify questions regarding women receiving the COVID vaccine at various stages in their lives, I would like to summarize some of the data and statements from the CDC (Center for Disease Control), ACOG (American College of Obstetrics and Gynecology), SMFM (Society for Maternal Fetal Medicine) and ASRM (American Society for Reproductive Medicine).
Infection with COVID has been proven to result in worse outcomes in pregnancy; these include, but are not limited to, preterm delivery, pregnancy loss, maternal ICU admissions and maternal death. Due to this, the American societies related to OBGYN, REI, MFM have taken a firm stance on vaccine recommendations for women.
The mRNA COVID vaccines (Moderna and Pfizer) and the Johnson & Johnson vaccine are found to be safe and are recommended for women to receive when given the opportunity. Using the data from thousands of women in pregnancy and during breastfeeding, it has been proven that there is no increased risk of miscarriage, adverse pregnancy outcomes or infant harm when the COVID vaccine is administered. Benefits of passive transfer of antibodies to infants in breast milk has been seen, which is beneficial for babies for COVID prevention. An immune response after the vaccine is common and these symptoms can include soreness at the injection site, redness at the injection site, fatigue, elevated temperature and chills. If these symptoms occur, it is safe to take Tylenol (acetaminophen) for symptom control, even during pregnancy. If you have a persistent high fever in the first trimester of pregnancy, it is recommended to take acetaminophen to lower your temperature.
For some women who have received the vaccine, a change in the menstrual cycle for 1-2 months can occur. This is not harmful and will resolve after the second vaccine dose. This cycle change does not cause any permanent damage and will not contribute to any difficulties with future pregnancies or future attempts to become pregnant.
If you have received the vaccine and plan to get a mammogram, it is important to notify your healthcare team about the dates you received your vaccine as some patients have temporary enlargement of lymph nodes as part of the immune response from the vaccine. This does not hinder your mammogram results and does not prevent you from getting your mammogram. This does not contribute to the development of breast cancer. The lymph node response is temporary and will resolve.
Even if you have already had COVID, it is important to get the vaccine as mutated COVID variants will continue to spread and your natural immune response is not as protective against COVID as the vaccine.
If you have questions or concerns about the COVID vaccine, please ask your healthcare provider so that these concerns can be addressed and you can make an informed decision about the vaccine.
The COVID-19 Vaccines Require Two Doses
Currently authorized vaccines, and most vaccines under development, require two doses of vaccine. The first injection helps the immune system recognize the virus, and the second strengthens the immune response. Both are needed – if both injections are not taken the vaccine you receive may not give you maximum protection.
If you have been diagnosed and recovered with COVID-19, the U.S. Centers for Disease Control and Prevention (CDC) recommends you still get the vaccine. A prior COVID-19 infection may not indicate immunity to the disease in the future.
Learn more about the benefits of getting vaccinated: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/8-things.html
Will the Vaccine Hurt or Make Me Sick?
The COVID-19 vaccines are administered as an injection into the muscle of the upper arm. A second dose is then administered either three or four weeks later depending on the vaccine.
There could be side effects, but they should go away within a day or so. Possible side effects include a sore arm, headache, fever, or body aches. This does not mean you have contracted COVID-19. Side effects are signs that the vaccine is working to build immunity.
Learn more about what side effects to expect and get helpful tips on how to reduce pain and discomfort after your vaccination: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html
Safety
1) KDMC is receiving and administering the COVID-19 vaccine from Moderna.
2) The Moderna COVID-19 Vaccine is an emergency use authorized product. It is not considered a FDA approved Vaccine.
3) Manufacturing and approval can be streamlined through an Emergency Use Authorization, which does not affect vaccine safety.
4) All vaccines are tested and reviewed by the Food and Drug Administration (FDA) to make sure they work and are safe.
5) The FDA reviews all safety data from clinical trials and authorizes emergency vaccine use only when the expected benefits outweigh potential risks.
6) The Advisory Committee on Immunization Practices (ACIP) reviews all safety data before recommending any COVID-19 vaccine for use.
7) FDA and CDC will continue to monitor the safety of COVID-19 vaccines.
How COVID-19 Vaccines Work in the Body
Vaccines expose the body to harmless molecules that mimic bacterial or viral infections so that our immune systems trigger and build resistance to actual infections. This tried-and-true approach prevents disease.
A new kind of vaccine
The COVID-19 vaccines developed by Pfizer and Moderna are a new type of vaccine, called an mRNA vaccine (messengerRNA). Instead of exposing the body to a weakened version of COVID-19, mRNA vaccines send cells a genetic message or instructions for the body to make a protein that triggers an immune response.
Who Approves Vaccines?
KDMC is receiving and administering the COVID-19 vaccine from Moderna. The Moderna COVID-19 Vaccine is an Emergency Use Authorized product. It is not considered a FDA approved Vaccine.
In the U.S., all vaccines must adhere to the FDA’s high safety and quality standards. There is no official timeline for developing a vaccine, and in a public health emergency, manufacturing and approval can be streamlined through an Emergency Use Authorization, which does not affect vaccine safety.
Vaccines start with research
Vaccines work by safely mimicking the infectious bacteria or virus that causes disease. This stimulates our immune systems and builds up resistance so if we are exposed to the disease, our bodies know how to fight it.

Testing Is Essential for Safety
Clinical trials are conducted in three phases.
In Phase 1, the vaccine is given to a small number of generally healthy people to assess its safety and effectiveness.
In Phase 2, the vaccine is given to hundreds of people with different health conditions and from diverse demographic groups.
In Phase 3, the vaccine is administered to thousands of people across demographic groups and immune responses are compared against placebos, which are doses that don’t contain any of the vaccines and are used for testing purposes only.
